Page 870 - TNFlipTest
P. 870
OB30 Obstetrics
Medical Complications of Pregnancy
Toronto Notes 2019
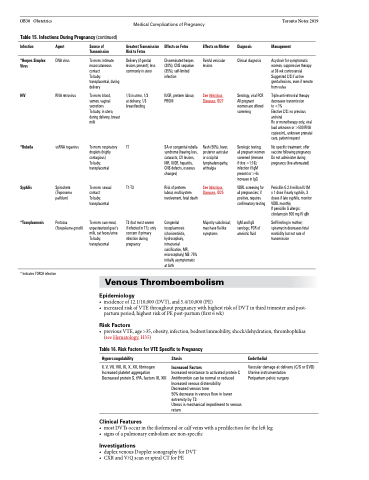
Table 15. Infections During Pregnancy (continued)
Infection
*Herpes Simplex Virus
HIV
*Rubella
Syphilis
*Toxoplasmosis
Agent
DNA virus
RNA retrovirus
ssRNA togavirus
Spirochete (Treponema pallidum)
Protozoa (Toxoplasma gondii)
Source of Transmission
To mom: intimate mucocutaneous contact
To baby: transplacental, during delivery
To mom: blood, semen, vaginal secretions
To baby: in utero, during delivery, breast milk
To mom: respiratory droplets (highly contagious)
To baby: transplacental
To mom: sexual contact
To baby: transplacental
To mom: raw meat, unpasteurized goat’s milk, cat feces/urine To baby: transplacental
Greatest Transmission Risk to Fetus
Delivery (if genital lesions present); less commonly in utero
1/3 in utero, 1/3 at delivery, 1/3 breastfeeding
T1
T1-T3
T3 (but most severe if infected in T1); only concern if primary infection during pregnancy
Effects on Fetus
Disseminated herpes (20%); CNS sequelae (35%); self-limited infection
IUGR, preterm labour, PROM
SA or congenital rubella syndrome (hearing loss, cataracts, CV lesions, MR, IUGR, hepatitis, CNS defects, osseous changes)
Risk of preterm
labour, multisystem involvement, fetal death
Congenital toxoplasmosis (chorioretinitis, hydrocephaly, intracranial calcification, MR, microcephaly) NB: 75% initially asymptomatic at birth
Effects on Mother
Painful vesicular lesions
See Infectious Diseases, ID27
Rash (50%), fever, posterior auricular or occipital lymphadenopathy, arthralgia
See Infectious Diseases, ID25
Majority subclinical; may have flu-like symptoms
Diagnosis
Management
Acyclovir for symptomatic women, suppressive therapy at 36 wk controversial Suggested C/S if active genital lesions, even if remote from vulva
Triple anti-retroviral therapy decreases transmission
to <1%
Elective C/S: no previous antiviral
Rx or monotherapy only, viral load unknown or >500 RNA copies/mL, unknown prenatal care, patient request
No specific treatment; offer vaccine following pregnancy Do not administer during pregnancy (live attenuated)
Penicillin G 2.4 million IU IM x 1 dose if early syphilis, 3 doses if late syphilis, monitor VDRL monthly
If penicillin G allergic: clindamycin 900 mg IV q8h
Self-limiting in mother; spiramycin decreases fetal morbidity but not rate of transmission
Clinical diagnosis
Serology, viral PCR All pregnant women are offered screening
Serologic testing; all pregnant women screened (immune if titre >1:16); infection if IgM present or >4x increase in IgG
VDRL screening for all pregnancies; if positive, requires confirmatory testing
IgM and IgG serology; PCR of amniotic fluid
* Indicates TORCH infection
Venous Thromboembolism
Epidemiology
• incidenceof12.1/10,000(DVT),and5.4/10,000(PE)
• increasedriskofVTEthroughoutpregnancywithhighestriskofDVTinthirdtrimesterandpost-
partum period; highest risk of PE post-partum (first 6 wk)
Risk Factors
• previousVTE,age>35,obesity,infection,bedrest/immobility,shock/dehydration,thrombophilias (see Hematology, H35)
Table 16. Risk Factors for VTE Specific to Pregnancy
Hypercoagulability
II, V, VII, VIII, IX, X, XII, fibrinogen Increased platelet aggregation Decreased protein S, tPA, factors XI, XIII
Clinical Features
Stasis
Increased Factors
Increased resistance to activated protein C Antithrombin can be normal or reduced Increased venous distensibility
Decreased venous tone
50% decrease in venous flow in lower extremity by T3
Uterus is mechanical impediment to venous return
Endothelial
Vascular damage at delivery (C/S or SVD) Uterine instrumentation
Peripartum pelvic surgery
• mostDVTsoccurintheiliofemoralorcalfveinswithapredilectionfortheleftleg • signsofapulmonaryembolismarenon-specific
Investigations
• duplexvenousDopplersonographyforDVT • CXRandV/QscanorspiralCTforPE